FastPMCFTM manages end-to-end cycle of the PMCF survey and enables the medical device manufacturers to get the feedback on clinical evidence from market in a quick turnaround time.
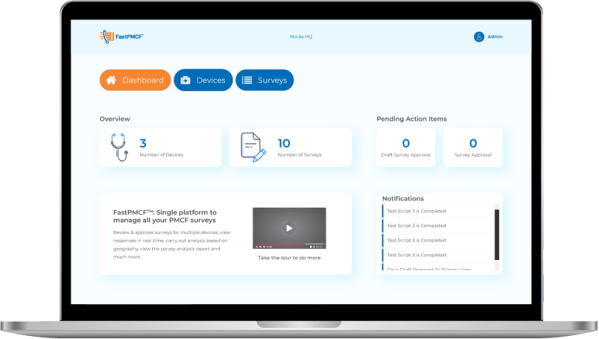
Single Platform to manage all your PMCF Surveys
FastPMCFTM enables medical device manufacturers manage their PMCF needs and collect real world evidence in a quick turn around time. The platform is uniquely designed to manage multiple surveys and ensure a smooth data capture process.
Key features

Manage survey(s) for multiple devices on a single platform
Surveys of all variants of devices in your portfolio can be tracked to help you prioritize per deadlines of audits
Custom questionnaire per PMCF plan
Get flexibility to create various types of questions per PMCF plan of the device and requirement of survey analysis
User friendly dashboard and real-time status for manufacturers
View the progress of survey starting from creation of questions, approval, launch, receipt of responses, dynamic analysis and survey completion.
Survey analysis report based on geography
Get dynamic survey analysis reports with slice and dice based on geography and profession of the survey participants to help you take critical decisions on the performance & safety of device
Unique link ensures data integrity
A unique survey link for each participant ensures data integrity and restricts each participant to submit survey response only once.
Launch survey to large number of participants in a single click
Get rid of travels and in-person meetings by sending the survey to bulk participants. Also reduce your lead time of conducting PMCF surveys.
Data encryption at rest and on the fly
Ensure data security with encrypted data of survey questions, device details, participant details, survey responses and survey analysis